
BÜHLMANN Laboratories AG, is proud to announce it received United States Food and Drug Administration (FDA) 510(k) clearance of a fecal extraction device for use in conjunction with the automated calprotectin test, BÜHLMANN fCAL®turbo.
The BÜHLMANN fCAL® turbo is an in vitro diagnostic assay intended for the quantitative measurement of fecal calprotectin, a neutrophilic protein that is a marker of intestinal mucosal inflammation, in human stool. The BÜHLMANN fCAL® turbo aids in the diagnosis of inflammatory bowel disease (IBD), specifically Crohn’s disease (CD) and ulcerative colitis (UC) and aids in the differentiation of IBD from irritable bowel syndrome (IBS) in conjunction with other laboratory and clinical findings.
Simpliyfing Extraction- Delivering
Speed, Quality, Flexibility in
Stool Pre-Analytics
BÜHLMANN CALEX® Cap is a fecal extraction device that offers an efficient, convenient and hygienic stool extraction. Its unique design makes it an ideal extraction device characterized not only by high safety but also by offering high stability of stool samples. Furthermore, the CALEX® Cap is fully compatible with total laboratory automation (TLA) solutions minimizing the complexity of sample processing thus significantly reducing hands-on time.
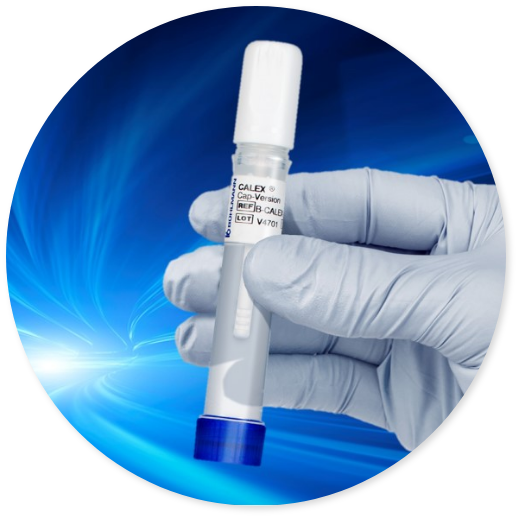
Speed
- Save time with a simple and convenient extraction procedure
Quality
- High correlation to gold standard manual weigh extraction method
Flexibility
- Can be applied directly on most chemistry platforms streamlining your workflow *

CALEX® Cap Benefits
- Ease of use for laboratory personnel and patients
- Delivers a precise amount of fecal sample
- Optimal dilution is achieved
- Can be used directly on many automated ELISA systems and many clinical chemistry analyzers
- Extract is stable for 3.5 days at 2-8°C
- CALEX® Cap offers precise stool extraction within 10 minutes
CALEX®Cap on Clinical Chemistry Analyzer
BÜHLMANN fCAL turbo® + CALEX® Cap = Total Laboratory Automation
Streamline Your Workflow, Reduce Hands-on Time
Get in touch



