BÜHLMANN Laboratories AG, is proud to announce that it has received United States Food and Drug Administration (FDA) 510(k) clearance of its BÜHLMANN fCAL® ELISA.
The BÜHLMANN fCAL® ELISA is an in vitro diagnostic assay intended for the quantitative measurement of fecal calprotectin in human stool. The BÜHLMANN fCAL® ELISA aids in the diagnosis of inflammatory bowel disease (IBD), specifically Crohn’s disease (CD) and ulcerative colitis (UC) and aids in the differentiation of IBD from irritable bowel syndrome (IBS) in conjunction with other laboratory and clinical findings.
Accurate, Precise, Efficient
BÜHLMANN fCAL® ELISA has rock-solid performance that provides you with optimal precision to measure your results. Focus on what really matters – calprotectin results you can stand behind. Increase your efficiency and cost savings with the most sensitive fCAL assay the FDA has registered.
- Accurate:
- Efficient: simple procedure, ready-to-use-reagents, results you can count on
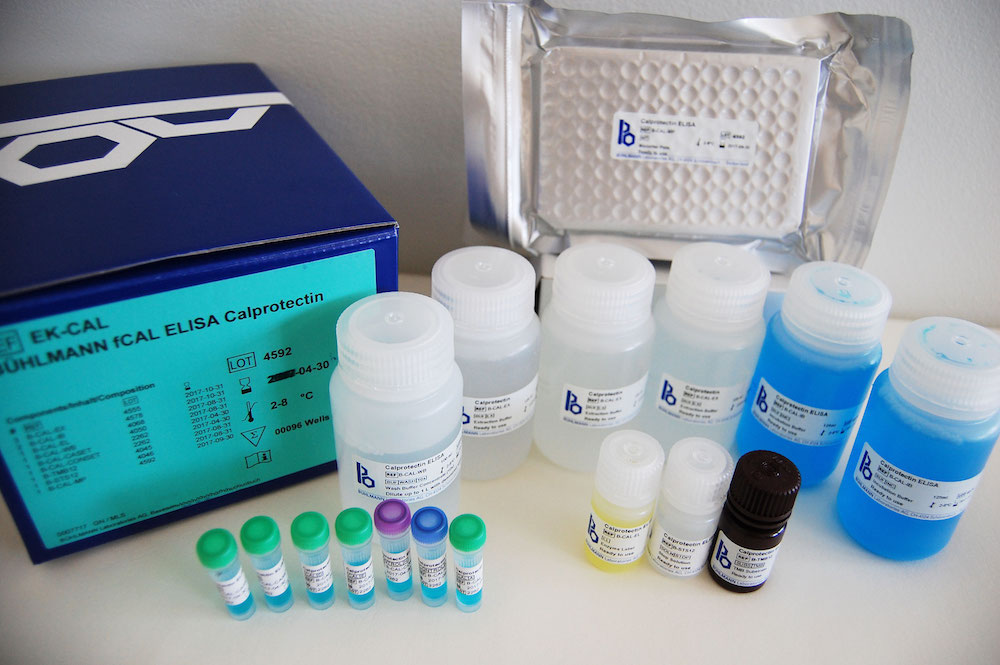
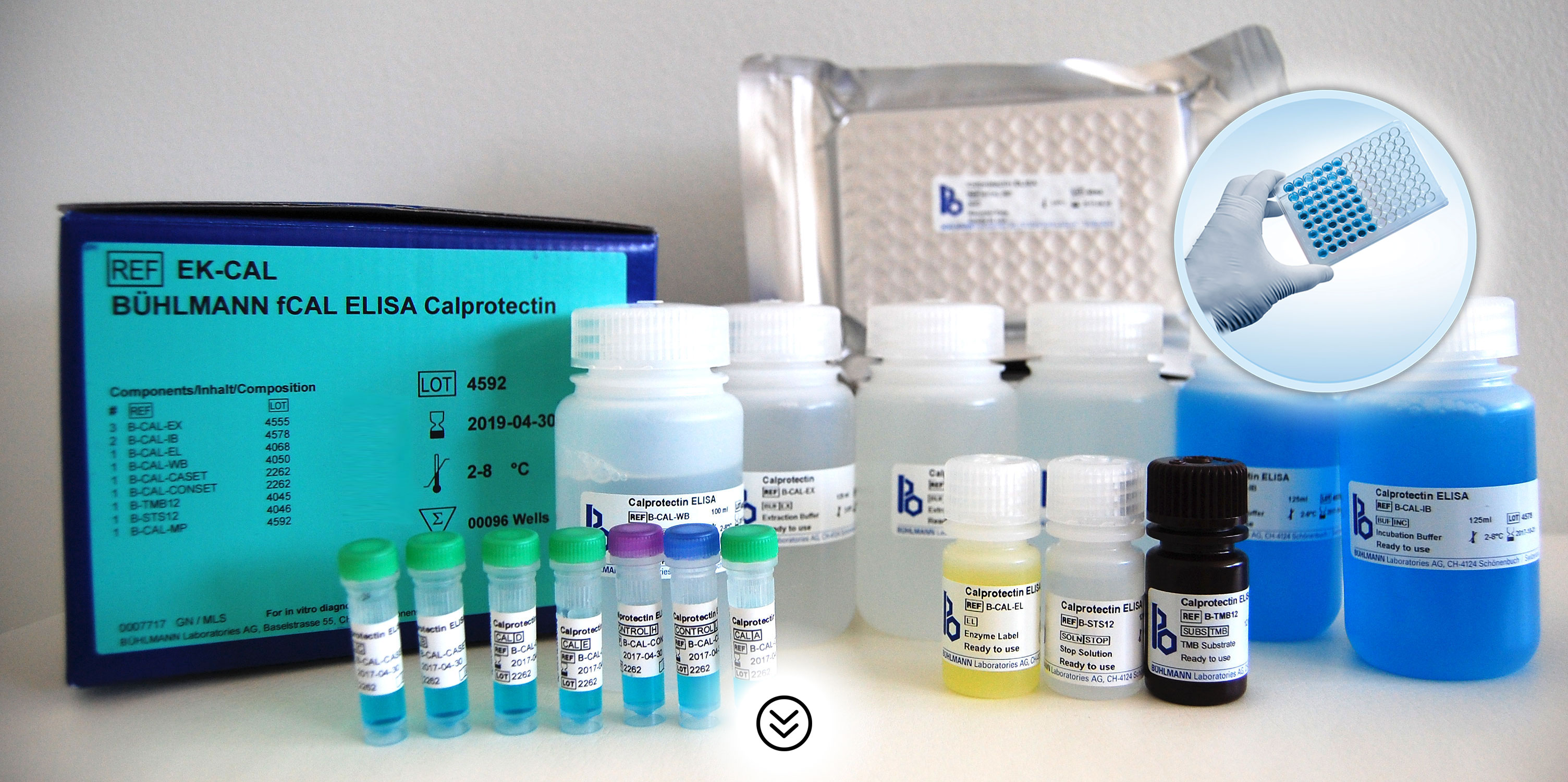
BÜHLMANN fCAL® ELISA Benefits
- Easy-to-use ELISA kit (total assay time is 75 minutes)
- Reagents are ready to use*
- Kit includes calibrators and controls
- Analytical Sensitivity: <30µg/g Calprotectin in fecal sample
- Measurable range 30-1800µg/g
*except wash buffer
BÜHLMANN fCAL® ELISA Citations

Choose BÜHLMANN fCAL® ELISA Because Accuracy is What Matters
Robust, Validated, and Published
Get in touch