BÜHLMANN fCAL®
Assays
Multiple Applications, Evolving Assays for Inflammation to Meet Your Needs
Trust Your Calprotectin Results – Robust, Validated and Published
Which Assay is Best for You?
The BÜHLMANN Calprotectin Assay product line has a range of solutions that services all types of users, from the subject’s home test to the high throughput labs running thousands of samples. We have a variety of test formats to best suit your needs and no matter which assay you choose results are reliable, accurate, and consistent across all the platforms.
Unique in Speed, Quality, Flexibility
For the highest throughput laboratories and can be applied on most routine clinical chemistry analyzers.
Health Canada Licence: 96808
Accurate, Precise, Efficient
For higher throughput, batch testing laboratory use (automation available on the multiple ELISA Processing Systems).
Health Canada Licence: 80726
Rapid and Quantitative
Lateral Flow Assay for Calprotectin and Therapeutic Drug Monitoring for low throughput use.
Health Canada Licences: Quantum Blue® fCAL: 83477, Quantum Blue® fCAL extended: 100161
US: Research Use Only. Not for use in diagnostic procedures in the US

BÜHLMANN fCAL® turbo
Speed, Quality, Flexibility
BÜHLMANN fCAL® turbo is a flexible turbidimetric assay for fecal calprotectin applicable on many major clinical chemistry platforms.
The technology of immunoturbidimetry implements a further milestone in fecal calprotectin quantification. It allows very rapid and flexible random access use, as well as being the ideal solution for high throughput applications in the routine laboratory.
The BÜHLMANN fCAL® turbo is a turbidimetric immunoassay based on the standardization of the BÜHLMANN fCAL® ELISA, which is globally established in laboratories as the gold standard for calprotectin measurements.
Speed: time-to-first-result is within 10 minutes
Quality: This assay is standardized against the well established BÜHLMANN fCAL® ELISA and the PETIA methodology enhances precision and reproducibility.
Flexibility: Immunoturbiometric assays can be applied to most clinical chemistry platforms, allows random access workflow which can be directly integrated into the existing laboratory infrastructure.
Range: 30 – 10,000 μg/g
Method Comparison
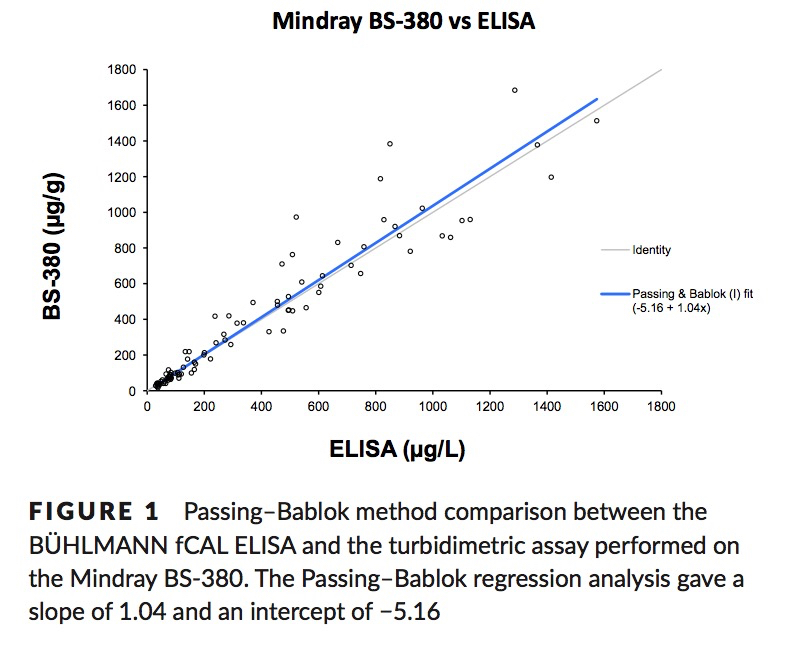
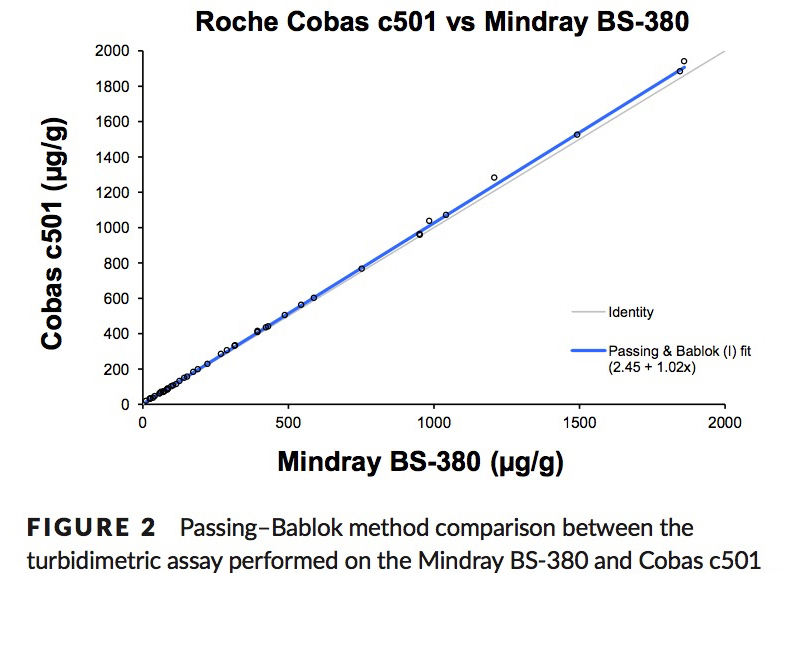
Nilsen, T. et. al. A novel turbidimetric immunoassay for fecal calprotectin optimized for routine chemistry analyzers. J Clin Lab Anal. 2016 Sep 15. PMID:27629827
Method Comparison: fCAL® ELISA vs fCAL® turbo
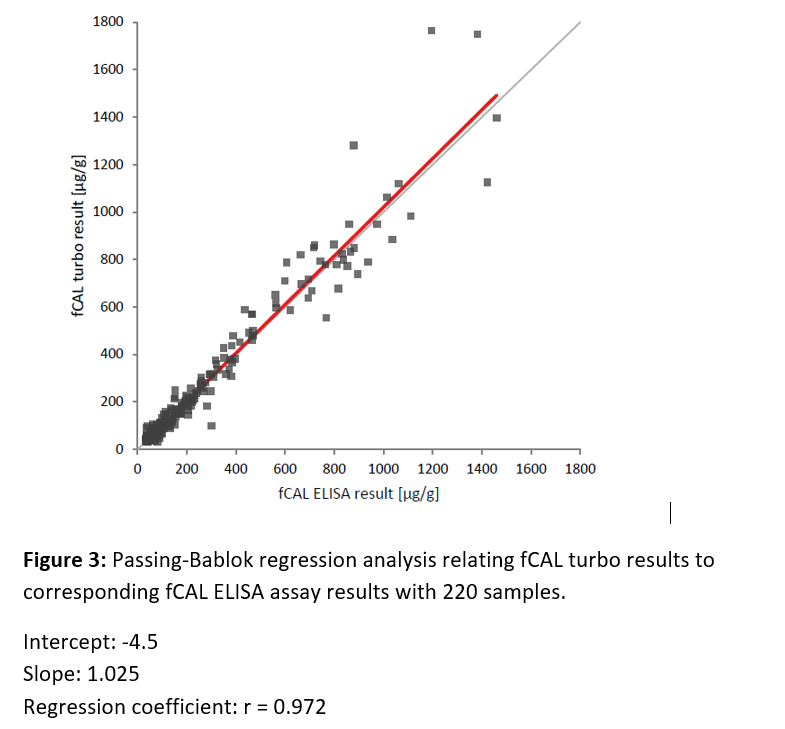
K. Sunde1, M. Schneider2, T. Nilsen1, Ch. Niederberger2, Th. Jermann2, E. Sunderhagen
1Gentian AS, Moss (Norway)
2BÜHLMANN Labortories AG, Schönenbuch (Switzerland)
Learn more about BÜHLMANN fCAL® turbo

BÜHLMANN fCAL® ELISA
Accurate, Precise, Efficient
The kit format is a microtiter plate based assay that can be performed manually or on an automated ELISA system to reduce hands-on time.
All reagents, standards and controls are provided in one kit and are ready for use.
There is no requirement for reconstituting any component, apart from the wash buffer.
This minimizes any errors as well as ensuring a long shelf life.
- Easy-to-use ELISA kit (total assay time is 75 minutes)
- Reagents are ready to use*
- Kit includes calibrators and controls
- Analytical Sensitivity: <30µg/g Calprotectin in fecal sample
- Measurable range 30-1800µg/g
* except wash buffer
Learn more about BÜHLMANN fCAL® ELISA
Quantum Blue®
Rapid and Quantitative
- BÜHLMANN’s Quantum Blue® Product line is designed with an innovative solution based on established lateral flow technology which utilizes highly specific monoclonal antibodies to capture the molecule being measured. Optical densities of test and control lines are detected by the Quantum Blue® Reader , (small, dedicated reading device) and translated into a quantitative result.
- The Quantum Blue® Calprotectin reader analyzes the signal intensity from the test and control line to give a quantitative value and is standardized with the BÜHLMANN fCAL® ELISA.
Why Use Quantum Blue®?
- Provides a quantitative measurement of calprotectin in fecal samples
- Simple and rapid extraction and test process
- Results within 12 minutes
- Excellent correlation with the BÜHLMANN fCAL® ELISA
- Based on established lateral flow technology
- Two kit formats available dependent on test range required:
Quantum Blue® Reader
4 Easy Steps
View this demonstration of CALEX® Cap extraction and quantitative fecal calprotectin measurement using the Quantum Blue® fCAL rapid test technology.
Comparison of Quantum Blue® to BÜHLMANN fCAL® ELISA
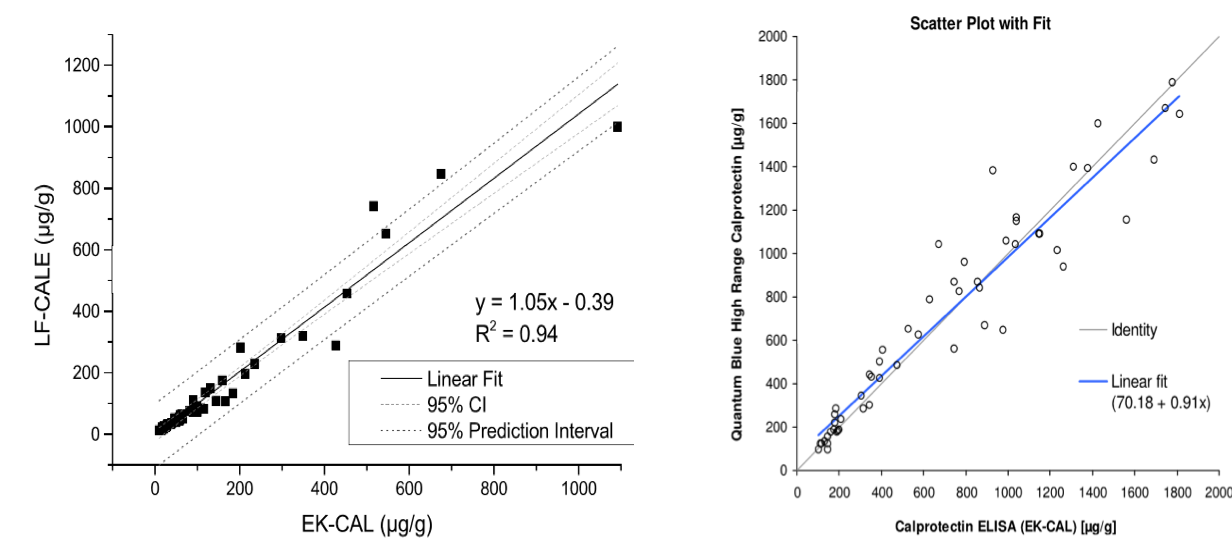
Assaying fCAL on Quantum Blue® LF test cassette/ cartridges (two options):
| Test | Quantum Blue® Calprotectin High Range | Quantum Blue® Calprotectin extended |
|---|---|---|
| Order Code | LF-CHR25 | LF-CALE25 |
| # tests per kit | 25x | 25x |
| Standard Range | 100-1800 µg/g | 30 -1000 µg/g |
| > top standard | Dilute and re-run or report >1800 | report as > 1000 |
| Final Dilution | *1:5000 | *1:500 |
| Reagents | Quantity | Code | Comments |
|---|---|---|---|
| Test Cassette | 25 cartridges | B-CAL-TC | vacuum-sealed in a foil bag |
| Extraction Buffer | 1 bottle 125 mL | B-CAL-EX | Ready to use |
| RFID Chip Card | 1 piece | B-CHR-RCC | Red plastic card |
| Reagents | Quantity | Code | Comments |
|---|---|---|---|
| Test Cassette | 25 cartridges | B-LFCALUS-TC | vacuum-sealed in a foil bag |
| Extraction Buffer | 1 bottle 125 mL | B-CAL-EX | Ready to use |
| RFID Chip Card | 1 piece | B-CALE-RCC | White plastic card |
| RFID Chip Card | 1 piece | B-CALE-RCC720 | Green plastic card |
Learn more about Quantum Blue®
IBDoc®
Three Easy Steps to Help Monitor IBD
- Process the sample with the CALEX® Valve extraction device.
- Apply the extracted sample to the calprotectin cassette.
- Use the smartphone CalApp® to read and calculate the result and send it to the gastroenterologist via the secure IBDoc® Portal.
- The BÜHLMANN IBDoc®, provides sophisticated technology which enables your IBD patients to perform quantitative calprotectin testing themselves, in just three easy steps, reading results using their smartphones.
- Process the sample with the CALEX® Valve extraction device.
- Apply the extracted sample to the calprotectin cassette
- Use the smartphone CalApp® to read and calculate the result and send it to the gastroenterologist via the secure IBDoc® Portal.
- IBDoc®provides a practical way for more frequent monitoring of patients in the privacy of their own home.
- Under the guidance of clinicians, more regular calprotectin monitoring can help to assess if patients are in prolonged IBD remission and serve as red-flag for increased calprotectin concentrations. This approach allows for a customized, patient-centric approach for IBD disease management.
- IBDoc® is the first self testing application for fecal calprotectin in IBD patients. Based on BÜHLMANN’s long lasting experience with calprotectin assays, IBDoc®allows IBD patients for convenient self testing of fecal calprotectin. CALEX® Valve has been developed for simple and easy-to-handle stool extraction. The incorporated valve allows the precise application of 60 µl onto the test cassette. The CalApp® smart phone application is available for iOS and Android transforms smartphones into a test cassette reader.
- BÜHLMANN IBDoc®is the first CE-marked home application for measuring the fecal inflammation marker calprotectin and has been developed based on BÜHLMANN’s long lasting experience in calprotectin assays.
- For further information, visit www.ibdoc.net or view the IBDoc® videos
Not available for Sale in the US.